Introduction
The goal of this article is to give you a practical understanding of Lead Acid batteries. We won't address the underlying chemistry, we'll treat them as a black-box and we will discover their characteristics and how to keep them healthy.
Disclaimer
I'm an amateur. I have absolutely zero relevant background in battery technology or electronics. I just scraped some information together in a hopefully useful manner.
A high-level overview of the lead acid battery
- It can provide a ton of current / power
- It hates to be deep-discharged and will die quickly if done repeatedly
- It hates being in a discharged state
- Only use 50% of total capacity if longevity matters (ideally only 30%)
- It's usable capacity depends on the load
- They are slow to charge (8-12 hours)
- They don't perform as well in cold weather
Lead acid batteries can provide a lot of current
Lead acid batteries can put out so much current that you can use them to weld2. They are widely used in ICE cars to power the starter motor, which needs hundreds of amps at 12 volt to turn over the engine.
They are also used to power mobility scooters, golf carts, trolly motors, small toy cars for children to ride in, or provide electricity on boats, caravans and in RVs. You can also find them in more stationary applications such in UPS systems1 or - of course - solar battery banks.
Danger
Lead acid batteries typically don't have any kind of short-circuit protection build-in. This means that if you (accidentally) short-circuit a lead acid battery, the battery can explode or it can cause a fire. Whatever object caused the short-circuit, will probably be destroyed.
Because lead acid batteries can supply such high currents, it's important to assure that you use the right wire thickness / diameter. If the wire is too thin, it causes too much resistance and thus may overheat, causing the insulation to catch fire.
Lead acid batteries can be very dangerous, so you have to be very carefull with them. Personally, I always make sure that anything connected to a lead acid battery is properly fused.
Lead acid batteries hate being deep discharged
The common rule of thumb is that a lead acid battery should not be discharged below 50% of capacity, or ideally not beyond 70% of capacity. This is because lead acid batteries age / wear out faster if you deep discharge them.
The most important lesson here is this:
Although a lead acid battery may have a stated capacity of 100Ah, it's practical usable capacity is only 50Ah or even just 30Ah
If you buy a lead acid battery for a particular application, you probably expect a certain lifetime from it, probably in years. If the battery won't last this long, it may not be an economically viable solution.
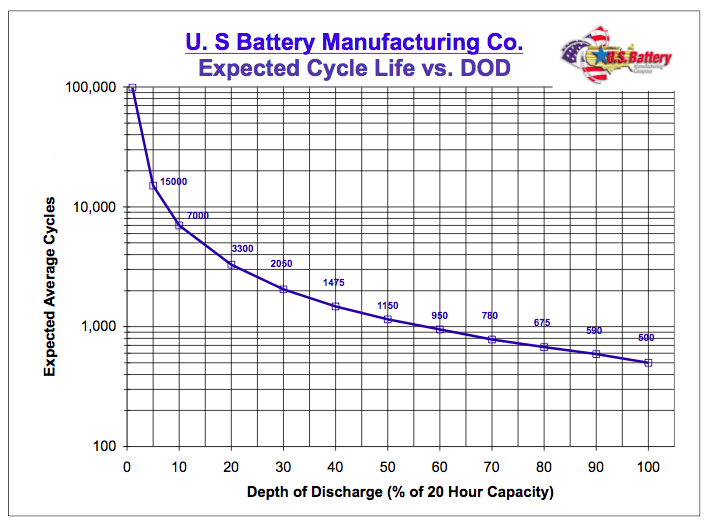
image source - Please note that this chart is based on a heavy-duty lead acid battery and doesn't reflect the lifecycle of a regular consumer lead acid battery. It is advised to look up the relevant chart for the particular battery model you may be interested in buying.
If you cycle a battery (with the characteristics depicted in the chart) every day as part of some kind of off-grid solar setup and you use 80% of it's capacity, you'll probably have to replace it after about two years.
If you add a few extra batteries in parallel, individual batteries may only be used 20% to 30% of capacity, and those same batteries may last 6 - 9 years. So by spending 2 or 3 times the money on batteries, you get 3 to 4 times the lifetime out of your setup.
So, for example, if you really need 100Ah of battery capacity, you may need two 100Ah batteries in parallel to assure longevity. You even may decide to buy three 100Ah batteries just to assure that they will last for the desired number of cycles.
However, if the battery setup is only meant for emergency power and thus only expected to operate a few times a year, discharging a lead acid battery to 80% of capacity is not a big deal. There is no need to add extra battery capacity because the number of charge/discharge cycles is so low that there isn't that much wear on the battery.
Lead acid batteries eventually die from old age
A lead acid battery deteriorates just by ageing. So even if it's kept full charged most of the time, it will wear out and needs to be replaced after a few years. It doesn't matter how well you treat them, even with the best care, they need to be replaced eventually.
Lead acid batteries hate being in a discharged state
Lead acid batteries should never stay discharged for a long time, ideally not longer than a day. It's best to immediately charge a lead acid battery after a (partial) discharge to keep them from quickly deteriorating.
A battery that is in a discharged state for a long time (many months) will probably never recover or ever be usable again even if it was new and/or hasn't been used much.
Usable capacity depends on the load
A typical 12-volt battery has a rating stated in ampere hour that tells you the capacity. For example, a battery can be rated as 70Ah.
So this could mean that the battery can sustain a load of 7A for 10 hours or 70A for one hour, right?
Unfortunately, no
It turns out that the usable capacity of a lead acid battery depends on the applied load. Therefore, the stated capacity is actually the capacity at a certain load that would deplete the battery in 20 hours.
This is concept of the C-rate. 1C is the theoretical one hour discharge rate based on the capacity. Batteries are mostly sold with a capacity based on a 0.05C discharge rate for 20 hours.
The C-rate is important because the C-rate is related to the usable capacity of a battery. That 70Ah capacity rating is based on a 0.05 C-rate or 20-hour discharge rate. That would be 70Ah / 20 = 3.5A.
This is important to understand: if you would put a higher load on this battery, the usable capacity will be less than 70Ah. For example, with a 7A load, the usable capacity may only be 64Ah (fake number for illustration purposes).
It also works in your favor: if the load is less than the 0.05 C-rate, the actual usable capacity will be higher!
So why is this?
When you put a load on a battery, the voltage drops a bit. Higher loads cause larger voltage drops, or to put it differently: the battery 'struggles' to maintain voltage.
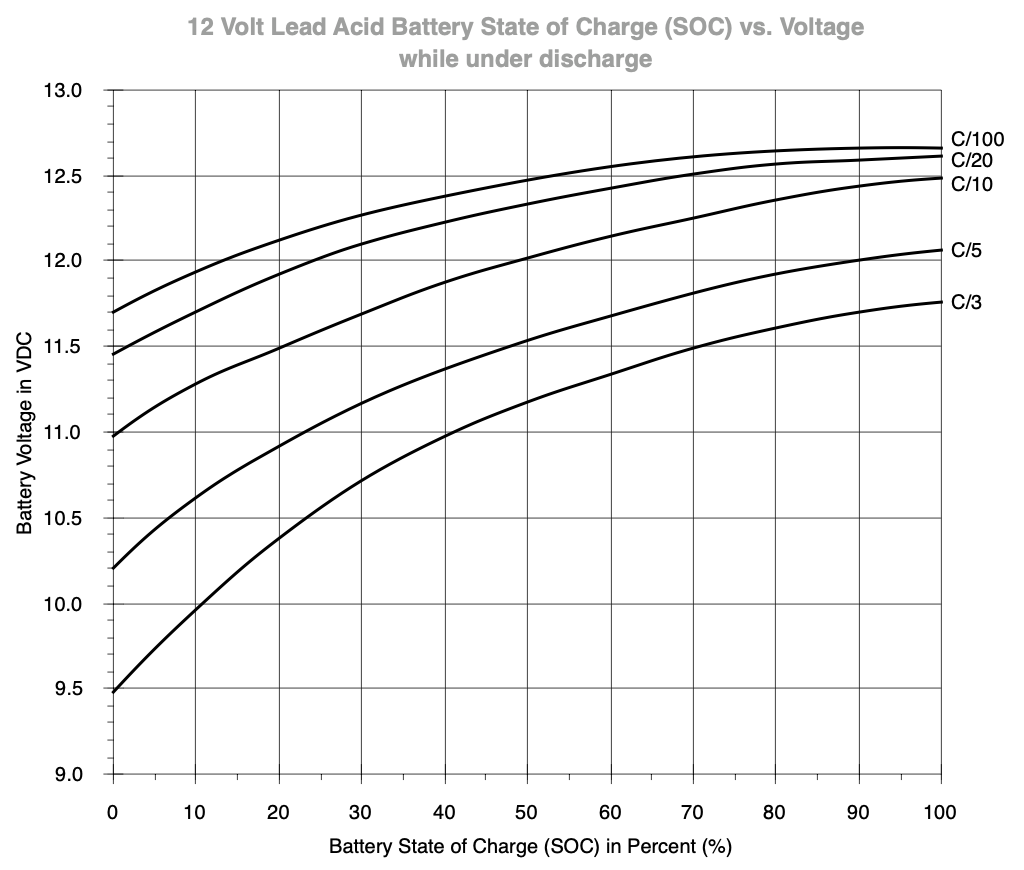
So if a load exceeds the standard 0.05C rate (C/20), you may have to select a higher capacity battery or accept a shorter run-time than you might expect based on the rated capacity on the label.
You even may consider putting multiple batteries in parallel to reach the desired usable capacity / runtime.
WARNING
The chart about the state-of-charge under load shows that you should keep an eye on the actual load and voltage. With a C/20 load, the battery is at 50% at 12.30 volt3.
A C/5 load on a 70Ah battery would be 14A. At that load, the battery is at 50% capacity at ~11.55 Volt under load. Only the load in combination with the voltage may give an indication of actual state-of-charge.
Predicting state-of-charge under load is doable with a static, constant load, but becomes more difficult when the load fluctuates, so take this into account.
ANOTHER WARNING
Different manufacturers produce different batteries that may have different discharge characteristics. This means that you should look up the battery specifications and hopefully find a discharge rate chart that will help you gauge actual capacity under load for this particular model.
How do you know the state of charge of a lead acid battery?
The state of charge is measured at rest: when the battery is not connected to any load or charger for 24 hours. The voltage will reflect the state of charge (SoC).
WARNING
There are many different, conflicting tables to be found on the internet that correlate voltage with a particular state of charge. Be sure you check that you pick the right one, consult the footnote4 for more information.
| State of Charge (SoC) | Voltage at rest (24h) |
|---|---|
| 100% | 12.70+ |
| 75% | 12.40 |
| 50% | 12.20 |
| 25% | 12.00 |
| 0% | 11.80 |
Please note that this table is only valid at an ambient temperature of 25C / 77F. If the temperature is lower, usable capacity diminishes and the voltages at wich a certain SoC is reached, will be higher.
Furthermore, these numbers can deviate a little bit depending on the kind of lead acid battery.
If you measure the voltage under load - for example, when you power some lights - the voltage does not reflect the actual state of charge.
It is quite difficult to determine the state of charge under load. Sometimes, battery manufactures provide a discharge chart that allows you to determine the state-of-charge based on the current load.
But often it is something you have to measure or figure out yourself. A constant load makes estimating battery capacity under load more predictable, but if the load varies, it is more difficult to accurately gauge the state of charge.
The positive impact on capacity of connecting batteries in parallel
By using multiple batteries in parallel, the load is also shared across all batteries. Each individual battery only has to supply a fraction of the total load. This means that in addition to the extra usable capacity of the added batteries, there is also added usable capacity because of the reduced load on each individual battery.
For example, if a 100Ah battery has a 0.05C discharge rate of 5A. If it has to provide 10A, the usable capacity is lower than the advertised 100Ah as explained earlier. If we add a second 100A battery in parallel, each battery now needs to supply only half of the load and thus will be able to provide the stated capacity as it is precisely the 0.05C discharge rate.
Lead acid batteries need deep discharge protection
It is highly recommended to use lead acid batteries in combination with a low-voltage cut-off solution that protects the battery against deep discharge5.

this article is not sponsored by victron
Ideally you can configure the cut-off coltage, such as with the depicted unit.
So many lead acid batteries are 'murdered' because they are left connected (accidentally) to a power 'drain'.
Charging a lead acid battery
No matter the size, lead acid batteries are relatively slow to charge. It may take around 8 - 12 hours to fully charge a battery from fully depleted. It's not possible to just dump a lot of current into them and charge them quickly. That would just overload and destroy the battery8.
Lead acid batteries need a specific 3-stage charge process6 in order to preserve their condition.
In practice, if you don't discharge a battery beyond 50%, it takes less time to recharge the battery7.
It can be a good idea to hookup unused batteries permanently to a 'tricklecharger'. This is a charger that charges the battery with a maximum current of 0.8A.
As it can take a very long time to charge a larger capacity battery with a tricklecharger, you need a regular charger, that can supply a decent current, to charge a battery 'within a reasonable timeframe'.
Lead acid battery types
Flooded / FLA
This is the well-known older type of battery. It may be necessary to add distilled water from time to time, so they require maintenance.
The key problem with batteries that require maintenance is that most people (consumers) don't know and if they know, they forget. These batteries basically don't match well with 'human nature'.
It seems to me that these batteries are on their way out in the consumer space, but are still prevalent in commercial/industrial application. It's probably easy for a business to just have a trained employee or service company periodically maintain the batteries.
EFB or Enhanced Flooded Battery
These batteries are improved versions of the regular flooded battery. They are more expensive, but will last more charge/discharge cycles, especially with deeper discharges.
Although not as performant as AGM batteries (which will be discussed shortly), they provide a cheaper alternative to AGM batteries.
Sealed Lead Acid
This type of battery is fully sealed. SLA batteries essentially the same as VRLA batteries but this name is used for the smaller capacity batteries, as found in motorcycles, uninterruptible power supplies and such.
These are maintenance-free batteries. They never require any maintenance during their lifetime. You don't need to add distilled water or anything during their lifetime.
Valve-Regulated Lead Acid
This name is used for batteries like the SLA battery, but with higher capacities. See also wikipedia. They have liquid inside like the flooded battery, but they are sealed and don't need any maintenance. To be precise: they can't be maintained, only be replaced.
The 'valve(s)' are only there in case of emergency, to release pressure due to gas buildup within the battery case if charged incorrectly.
AGM (Absorbent Glass Mat)
This is also a fully sealed SLA/VRLA battery, but it is even more advanced. They are better able to withstand deep discharges and can be recharged faster. This comes at a relatively steep price.
The faster recharge cycle can be important if used within a solar power bank, because there are only a limited number of hours when the sun provides enough energy for charging.
Deep-Cycle
These batteries are build differently9 and are less suited for starting cars, but better suited to provide power to power boats, RC vans or form a solar power bank.
They are often not a kind of battery in and of itself: there are just regular flooded deep-cycle batteries, or AGM deep-cycle batteries. They are often specifically designed for solar power banks or similar applications.
Evaluation
Although regular flooded batteries will have the longest lifespan of all lead acid battery technology, they require regular maintenance and that may not be practical. Therefore, AGM or other maintenance-free batteries are better suited for residential battery applications, the relatively lower life expectancy is just the price for practicality/convenience.
Low self-discharge rate and storing batteries
Lead acid batteries needs to be stored fully charged. They should be recharged at least every six months due to self-discharge, although the self-discharge rate is rather low.
Buyer beware - ask for fresh batteries
I've ordered quite a few smaller SLA batteries from various brands to test their capacities. I noticed that the actual brand didn't matter much. The age of the battery seemed to matter.

some of the tested SLA batteries
While they are in storage at the vendor, they are probably never recharged, which deteriorates the battery. The batteries with a lower SoC correlated with a serial number that indicated that they were older than the other batteries.
So it might be beneficial to specifically ask for a 'fresh' battery when you order a lead acid battery.
Q & A
Can my lead acid battery be revived?
No.
If the voltage of a 12 volt battery at rest is close to zero, it is dead.
There are tips like 'using epsomsalts' or keeping them on a charger for weeks, but at best, you get only a small portion of usable capacity back, if any. A battery 'revived' like this should never power something you rely on. Personally I don't think it's worth the cost of epsom salt or your time, but you have to decide for yourself if that's true or not.
If a battery is totally dead, I would recommend to accept the loss and get a new one.
The impact of cold weather on performance
If a lead acid battery is exposed to colder or even freezing temperatures, it will work fine, but it can output less current. This is relevant for older, more worn-down batteries. Such batteries can still work fine in the summer, but may no longer be able to start a car or provide another utility with sufficient power when temperatures drop significantly.
Does it make sense to use Lead acid batteries for an off-grid solar setup?
You can do a lead acid solar setup if you can get those batteries cheap but otherwise it may be better to go for a LiFePo4 based setup. Although the initial investment is much higher, Lithium-based batteries will be cheaper long-term because they last so much longer than lead acid batteries (life-time).
I think lead acid batteries are suited for climates with a lot of sunlight available all year round, to power a livingspace through the night.
Since lead acid batteries don't 'like' to be in a discharged state for a long time (more than a day at most), I don't think they are suitable for a more temperate climate, with lots of overcast days.
So the first issue with lead acid batteries is that they don't take well being in a discharged state for more than a day or so. It will make them deteriorate faster.
I think the second issue with lead acid batteries as a solar power bank is their slow charging speed. Lead acid batteries often can't use all available solar power to charge because they just can't charge any faster, no matter their capacity.
This means that even though there would have been enough energy available to fully charge the batteries, it was not available long enough to fully charge the batteries. Maybe AGM batteries may help as they can be charged with higher currents, even though they may not last as long.
Lithium-based batteries can be charged with very large currents and can - in some sense - capture every bit of sunlight that's available. This is much better suited to climates with more intermittent sunny days or even sunny hours, I think.
Another thing that comes to mind is that if you really want to go with lead acid batteries for a solar bank, flooded may be the longest lasting, but the regular maintenance they require may quickly become a chore / unmanageable. I have zero experience with this, but please verify this beforehand. All the more reason to consider at least maintenance-free lead acid batteries, even if they may not last as long.
This is just my thought, I'm no expert on this.
Just remember that regular car batteries are just not suitable for this application. You need - more expensive - batteries that are build specifically for being used in a power bank10.
Why are lead acid batteries so widely used in cars?
Cars need a power source that can provide a lot of power to run the starter motor. Starter motors can use anywhere from 1.5 to 3 Kilowatt when cranking the engine. That's about 125A to 250A of current at 12 volts.
You may notice that batteries are often rated for much higher CCA or 'Cold Cranking Amps' values, but since they deteriorate over time, that extra margin will come in handy. Especially in colder weather.
Lead acid batteries as used in cars can last many years because they are used under near ideal conditions. They are always kept fully charged and are ony briefly and slightly discharged. They are immediately recharged after the car is started.
How can I check if a battery is healthy ?
You need a battery tester for this. They can be had for around 50 Euro's, which is not far off from just buying a new battery, which you might have to do anyway.
A demonstration video of such a cheap charger.
-
A UPS can be quite small, to power just a single computer, running off a 'small' 12 volt 7Ah lead acid battery (depicted further down below in the acticle). A step up in size would be a 19-inch rackmounted UPS, which can often be expanded with multiple external battery packs. A datacenter scale UPS is build using many large batteries in both series for higher voltages and in parallel for higher capacity. Lead acid batteries are well-suited for these type of applications because they are always kept fully charged and rarely (fully) discharged. In datacenter applications, they often only need to last until the diesel generators kick in. ↩
-
Just because you can, doesn't mean you should. Don't do it. ↩
-
Notice the voltages in the C/20 discharge rate - which should reflect the numbers in the table shown earlier - are actually a bit higher. If you want to be safe, using higher voltages is always safer for battery longevity, but at the cost of usable capacity. ↩
-
This article goes into more detail about this. Be sure you look at a table that correlates resting voltage against SoC and not the voltage under load. If you see a table with 10.8 volts at 0%, you are looking at a table for under load voltages. A battery at 10.5 - 10.8 volts at rest is probably damaged. A lead acid battery should never be below 11.80 volt at rest. ↩
-
'bad' battery protection solutions will just start to oscillate as the battery voltage recovers (above the cut-off threshold) when the load is removed. I bought a cheap 20 Euro unit and it was effectively useless because of this problem. ↩
-
https://en.wikipedia.org/wiki/IUoU_battery_charging ↩
-
If Lithium-based batteries have one big upside over lead acid batteries in energy storage applications, it might be this aspect: they can be charged much faster. It may make sense to oversize the solar power array just to charge the batteries as quickly as possible within the limited number of available 'sun-hours'. ↩
-
It is critical that a proper battery charger is used. You should never just apply a static current as overcharging the battery may lead to the buildup of flammable gasses like hydrogen. There are many documented cases of car batteries exploding in this way. Not only can you get hurt by debris, the internal liquid is acidic which can cause significant burns and is especially dangerous for the eyes. ↩
-
They have ticker plates that are better able to withstand deep discharges at the cost of lower peak current. ↩
-
I myself do use regular car batteries as part of my solar-powered blog because I got them for free and even if they are shot, they may last for quite a bit. I can also imagine that people would actually build a battery bank made of old car batteries and just ad a whole lot of them, if you have the space. I'm not sure if that kind of setup would be quite reliable. ↩
-
The car batteries are free, and I had no other use for the gel batteries so I hooked those up too (in parallel). The batteries have wildly different capacities and this is absolutely not recommended. If you hook up batteries in parallel, always use the same capacity. ↩


Comments